Reaction rates big idea every chemical reaction proceeds at a definite rate but can be speeded up or slowed down by changing the conditions of the reaction.
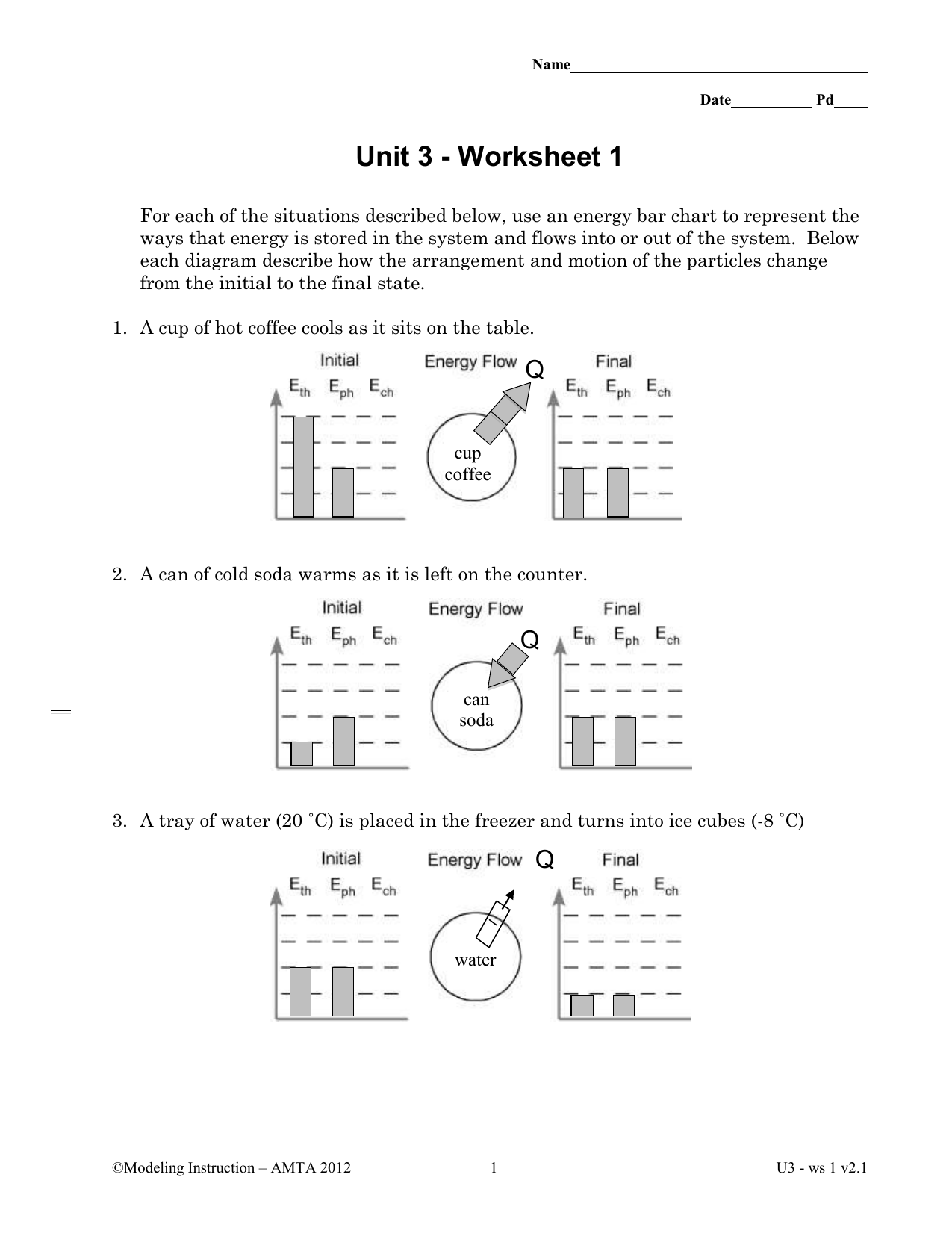
Energy ws 1 reaction rates.
Specify whether each reaction is exothermic exo or endothermic endo.
Reaction rates and potential energy diagrams 1.
Not all letters will be used a.
Reaction rates energy heat and temperature.
1 2 3 5 7 2 or seven halves order note.
The units of a rate constant will change depending upon the overall.
Rate law worksheet 2.
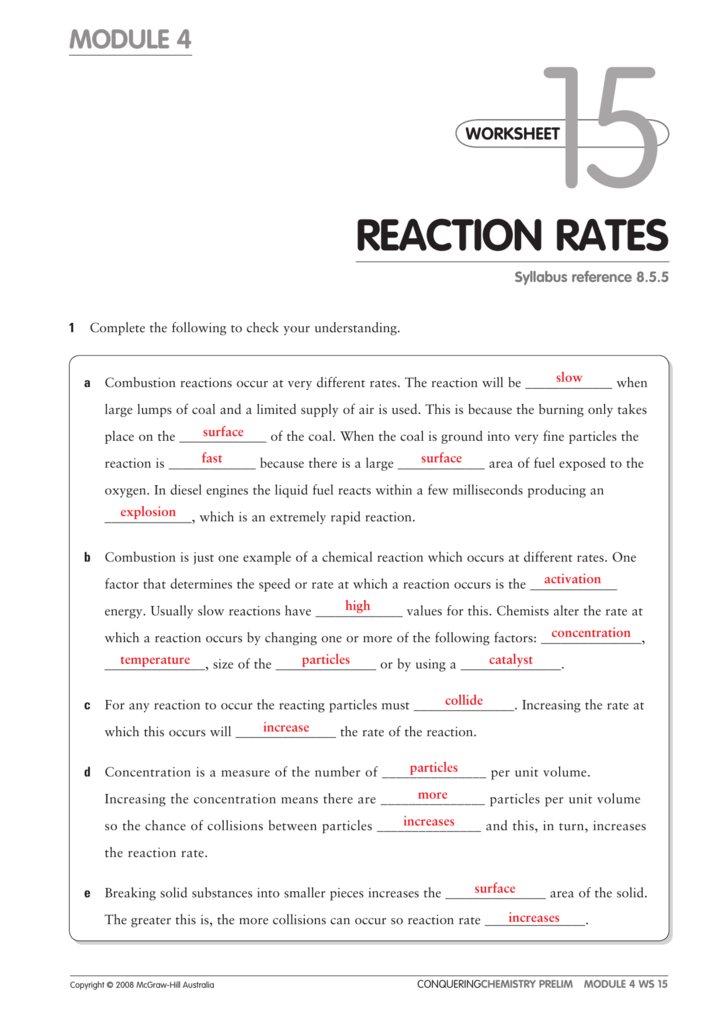
Worksheet 15 on kinetics answer key 1.
16 2 factors affecting reaction rates main idea factors.
If every collision between reactants leads to a reaction what determines the rate at which the reaction occurs.
Fill in the blanks on the reaction coordinate diagram with the appropriate letters.
Not all letters will be used reactants.
5 the overall order of a reaction is the sum of the individual orders.
Fill in the blanks on the reaction progress diagram with the appropriate letters.
16 1 a model for reaction rates main idea collision theory is the key to understanding why some reactions are faster than others.
Rate law worksheet 1.
Concentration and order of reactants and products x which increases as rate increases the activation energy.
The reaction shown in the diagram to the right.
Energy worksheet 1 reaction rates answers.
514 517 532 541 part a reaction energy 1.
Part a reaction energy.
When the order of a reaction is 1 first order no exponent is written.
What factors determine the rate of a reaction.
6 3 reaction rates and reaction mechanisms.
Energy is the ability to do or produce.
The law of conservation of energy states that energy can be from one form to another but can be neither nor.
For what reasons may a collision fail to produce a chemical reaction.
Summary sheet factors affecting reaction rates read p.
Collision theory pg 371 11 14.
How does each of them affect the rate.
Units for the rate constant.
Shown in this equation the factors affecting rate of reaction are.
Reaction energy rate ch.